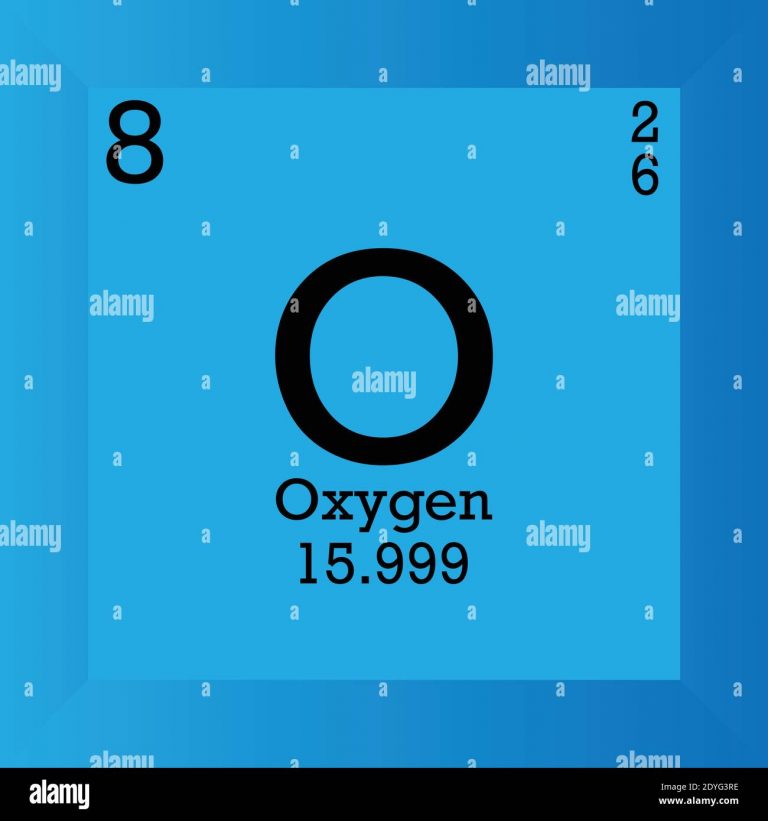
Oxygen is one of the most abundant elements in the universe and on Earth. However, it is important to know oxygen’s molar mass. The atomic number of oxygens is eight, and the molar mass of oxygen is about 15.9994. In this article we will try to understand what molar mass is and how it is related to doing calculations in chemistry. It will further help us to understand why knowing oxygen’s molar mass is important. We can also measure compounds using the concept of molar mass. For a molecule (for example, nitrogen, N2), the mass is equal to the sum of the atomic masses of the two nitrogen atoms. As for nitrogen, its atomic mass is simply (14.01 + 14.01) = 28.02 amu.
![]()
An element’s atomic mass is simply the sum of the atomic masses of all its individual atoms. This result is the molecular mass of a molecule. In other words, N2 has a molar mass of 28.02 grams per mole. When referring to compounds that are not molecular, the term “molecular mass” is inappropriate. So, we generally use the term “formula mass”. Ionic compounds do not have individual molecules. If we are talking about a mole of an ionic compound, we will still use the term molar mass. Therefore, the formula mass of calcium hydrogen carbonate is 117.10 amu and the molar mass of calcium hydrogen carbonate is 117.10 grams per mole (g/mol).
Molar Mass of Compounds
Every substance takes up some space and has certain mass (weight). Now, when doing any experiment, we often need to measure the weight of the molecules. The molecules make up substances, and it is important that we measure their weights accurately. But, how can we measure something so small in an accurate way? How to normally measure molecules? In the science laboratory, we use a tool called an analytical balance to measure molecules in grams.
Read Also: Acostarse Reflexive Conjugation- Present, Past
Through careful calculation, scientists can determine the number of moles a specific reaction will need. A mole is a unit of measure that helps us compare particles of any given substance and its mass. If we already know the number of moles we need, we can use the concept of molar mass to calculate how many grams of the substance are required. The molar mass, also known as molecular weight, is the sum of the total mass in grams of all the atoms that make up a mole of a particular molecule. The unit used to measure is grams per mole.
Why Molar Mass is Important
A chemical substance’s molar mass is the mass that one mole of that material has, or the mass that one mole of a given substance has. However, in the case of moles, this term isn’t very helpful. A mole is a unit of measurement for expressing the quantity of a substance.
Scientists measure the amount of elementary entities in a chemical sample in moles. Atoms and molecules are the fundamental building blocks of the universe. One mole of carbon 12 contains the same amount of elementary entities as there are atoms. As a result, Avogadro’s number (6.02214076 x1023) is the total number of atoms.
The mole and Avogadro’s number are fundamental ideas in chemistry. Chemical reactions include billions of atoms interacting with one another and rearrangement. Yet, representing billions of atoms would be difficult.
However, the scientists require a measurement unit that can represent billions of components. Moles are employed in chemical calculations because they represent 6.02214076×1023 atoms. We do it this way so that we can compare weight to the number of atoms in the substance (as defined by the number of moles). This is because weight is easier to track than the number of atoms in a chemical sample.
Each molecule of water is composed of one oxygen atom and two hydrogen atoms, so one mole of water is composed of a mole of oxygen and two moles of hydrogen. We can represent this relationship as following:
1 mole of H2O = 2 × 6.02214076 × 1023 of Hydrogen + 6.02214076 × 1023 of Oxygen.
In other words, the mass of one mole of a substance is equal to its molecular weight. For example, the molecular weight of water is 18.015 atomic mass units. While one mole weighs approximately 18.015 grams.
How to Find the Molar Mass for Compounds
Compounds are substances that are made up of more than one element. For example, some common compounds include salt, glucose, acetic acid (or vinegar), and sodium bicarbonate (or baking soda). Let’s see how to calculate the molar mass for the compounds. Let us use this compound, sodium chloride, for example. It is composed of two elements, sodium and chloride. The first thing we need to do is to find sodium and chlorine in the periodic table.
Step 1: Find the atomic masses of individual elements in the periodic table
The first thing we need is to find the individual atomic masses for each element. The element sodium has an atomic mass of 22.98976 g/mol. The element chlorine has an atomic mass of 35.453 g/mol.
Step 2: Count how many atoms there are for each element
For the compound sodium chloride, there are no subscripts. It means that there is only one sodium and only one chlorine atom for this compound.
Step 3: Find the molar mass
Now that we know how many atoms there are for each element, we can find the molar mass.
First, we calculate the mass of the sodium atoms, which is 22.98976 grams per mole. Next, we do the same for the mass of chlorine atoms, which is 35.453 grams per mole. Then, we add these two masses together to find the total mass of the molecules.
This comes out to 58.44276 grams per mole which we can round to 58.44 grams per mole. Now, in this article, we will try to find out the molar mass of oxygen molecules.
General Description of Oxygen
Oxygen is a colourless, odourless, and tasteless gas. It supports life on earth. It is non-flammable, but it actively supports the burning of combustible materials. Some materials that will not burn in air will burn in oxygen. Materials that burn in air will burn more vigorously in oxygen. As a non-liquid gas, it is shipped at pressures of 2000 PSIG or above. Pure oxygen is non-flammable. Production of synthesis gas from coal has the use of oxygen. It is used in CPR and as an inhalant.
Oxygen is an element displayed by the symbol O. Its atomic number is 8. It is an essential element for human survival. Decreased oxygen levels may be treated using medical oxygen therapy. Oxygen therapy is used to treat emphysema, pneumonia, some heart disorders, some disorders that cause increased pulmonary artery pressure, and any disease that impairs the body’s ability to take up and use gaseous oxygen. Under certain conditions, we can find higher quantities of oxygen than surrounding atmosphere.
Physical Properties of Oxygen
Nitrogen is less soluble in water than oxygen because it has about one molecule of oxygen for every two molecules of nitrogen. Oxygen’s solubility in water depends on its temperature. At 20°C, water dissolves half as much oxygen as it does at 0°C, dissolving 7.6 mg/L versus 14.6 mg/L. For one litre of fresh water at one standard atmosphere and 25°C, there is approximately 6.04 mL of oxygen. Under the same atmospheric conditions, seawater contains only 4.95 mL of oxygen per litre.
Oxygen freezes at 54.36 K (−218.79 °C, −361.82 °F), while it condenses at 90.20 K (−182.95 °C, −297.31 °F). As a result of the absorption of red wavelengths, oxygen has a light blue coloration both in its solid and liquid forms.
Chemical Properties of Oxygen
Oxygen has no odour, taste, or colour. Oxygen in the atmosphere is formed at regular/standard pressures and temperatures. According to the periodic table, oxygen belongs to the chalcogen group. Oxygen is a highly reactive element as the compounds of oxygen can be formed easily with most additional elements as well.
Oxygen has the second highest electronegativity of all the elements, after fluorine. It’s also a powerful oxidizer. After helium and hydrogen, oxygen is the element with the largest abundance in the universe. Approximately half the crust of the earth is also made up of oxygen. It is the most abundant element by mass in the crust.
We can only explain the presence of free oxygen on Earth by the photosynthesis carried out by living organisms. As plants use the energy of the sun and water to make usable energy, they produce elemental oxygen. However, diatomic oxygen just began to accumulate in the atmosphere approximately 2.5 billion years ago due to the appearance of photosynthetic organisms.
Molar Mass of Oxygen Molecule
An element’s molecular mass is defined as the sum of the masses of its elements. Now, how to calculate the molar mass of oxygen molecules? First, multiply the atomic mass of an element by the number of atoms in the molecule. After that add the masses of all the elements in the molecule.
An oxygen atom has a mass of 16 amu.
A molecule of O2 has a mass of 2 × 16 = 32 amu.
Mass of one molecule of oxygen
⇒ 32/ Avogadro constant (6.02214076 × 1023)
⇒ 5.31 × 10-23g.
The Importance of Molar Mass of Oxygen and Other Molecules
The molar mass of a material can be used to calculate how many moles are present in a sample of that substance. We need it to calculate its molecular mass. However, without knowing the molar mass of the substance, it is impossible to directly measure the number of moles in a sample.
We can calculate the molar mass of a substance by dividing its mass by its quantity. Usually, the result of this calculation is in grams per mole. There are about 47.88 atomic mass units in a mole of titanium, so its molar mass is 47.88 grams.
In grams per mole, the characteristic molar mass of an element is equal to its atomic mass. You can also determine the molar mass of a substance by taking the molar mass constant (1 g per mole), and multiplying it by the atomic mass. After that, you must sum up the atomic mass of each atom. This should give you the molar mass of a compound containing a variety of atoms.
As an example, if you want to find the molar mass of NaCl, you’ll need to find the atomic masses of sodium and chlorine. The atomic mass of chlorine is 35.45 g per mole, while the atomic mass of sodium is 22.99 g per mole. The result of combining these two masses is 58.44 g per mole.
F. A. Q s
What is the molar mass of Oxygen?
Ans. 15.999 u
What is the molecular mass of Oxygen?
Ans. Oxygen has 8 protons and eight neutrons. Therefore, its molecular mass is 16u. As the Periodic table shows, the atomic mass of O is just slightly less than 16. We can round it to 16. The oxygen molecule is O2, so it’s molecular mass is 2 X 16 = 32.
How to calculate the molar mass of oxygen gas?
Ans. For the Molar mass Of Oxygen Gas, we must know the Molar Mass of elemental Oxygen that is 16 g/mol. In order to calculate molar mass of oxygen gas, that is O2 , Multiply 16 with 2 and get 32g/mol that is molar mass of oxygen gas Molecule.
What is the difference between atomic mass and molar mass?
Ans. Molar mass is the mass of the one mole of the compound whereas atomic mass is the mass of the individual unit of the compound. Basically, molar mass is the mass of an average of many elements of the compound and atomic mass is the mass of the atom.
What is the difference between molar mass and molecular mass?
Ans. Molar mass refers to the mass of a mole in a substance, whereas molecular mass refers to the mass of a single molecule.