Just like we have a place to be living, in the earth of subatomic particles, electrons also have their own place. Where they go is said words to be taken down in writing by the electron form, which gives a detailed account of the electron order within an atom. For electrons, there is a get onto land state and an excited state. In this teaching event, we will be having a discussion about the get onto land state electron form.
When we talk about the get onto land state electron form, we are talking about the electron form of an atom at the lowest energy level possible. In the all existence, the natural state of a system is to use the lowest energy possible, so for atom 5’s electrons at the get onto land state, the electron order will be at the lowest energy state that is possible for that atom.
Get onto land state electron forms are the start for getting through knowledge smallest units joining together, properties, and structures. From the electrons in an atom, to the being unlike going round another and hybridization, the get onto land state electron form (puts in) rough building light on many different atomic properties. Fundamentally getting through knowledge electron form leads to a getting through knowledge of the periodic table.
Ground state electron configuration definition
The electrons that surround an atom are in a perfect arrangement. The electron configuration in the ground state tells us which orbitals will be present in a specific atom.
Read Also: The Molar Mass of Oxygen Molecule – Definition, Formula, and Example
The ground-state electron configuration, on the other hand, indicates the atom or molecule’s lowest conceivable energy configuration. This is why it is referred to as the ground state. It is the lowest possible level of energy. This configuration, on the other hand, is optimal for the atom. It is, nevertheless, the most straightforward electron arrangement to maintain. Now, the electrons’ positions in our atom reveal a great deal about their atomic structure. We now know what the ground state is and have a basic understanding of it.
The ground state electron configuration of an atom
In its neutral state, however, a silicon atom possesses 14 protons, neutrons, and electrons. The next step is to determine which orbitals silicon deposits its electrons in.
Occupancy Principles and Rules
There are rules and principles that must be followed in order for electrons to inhabit the lowest energy orbitals possible around the atom. Let’s take a closer look at those.
Aufbau principle:
If you have a lot of luggage and are staying in a hotel without an elevator, you will naturally choose a room on the ground floor that is closest to you. The Aufbau principle shows that electrons in the ground state behave in the same way. According to the Aufbau principle, electrons fill the lowest energy orbitals first, then the higher energy orbitals.
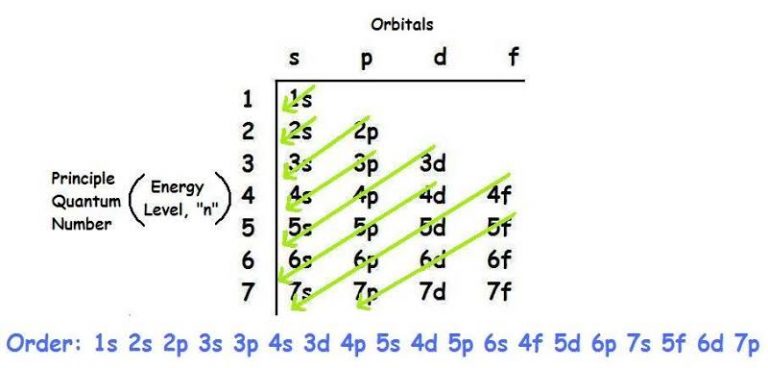
Diagram of the Structure
The energy level grows as you move upwards, with 1s having the lowest energy and 4p having the greatest, as indicated in the figure.
There are some exceptions to the Aufbau concept. Because fully or partially filled d orbitals are stable, rearranging the electrons in a d orbital with 4 or 9 electrons is required. An electron from the s orbital will transfer to the d orbital. A good example is chromium (CR):

Aufbau Principle: Exception
Another example is silver, whose symbol on the periodic table is Ag:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d9
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 4d10
Ground state electron configuration rules
Aufbau principle
- The first rule of ground-state electron configuration is the Aufbau principle. Now we must start with the lowest energy states and work our way up to the higher energy ones. The 1s orbital is the first or lowest ground state. Next, write the energy levels 1 2 3 4 5 in our first column. The orbital names are s,p,d. We write them in the first row. The s orbital can carry one set of spin paired electrons, while the p orbital can hold three sets. The f orbitals, on the other hand, contain five sets of spin paired electrons. Finally, the d orbital can accommodate seven spin-paired electron pairs (14 electrons). S, on the other hand, is just a sphere that surrounds our nucleus. P orbitals come in three dumbbell-like shapes.
- The p orbitals can carry 6 electrons because each dumbbell form can hold 2 electrons. The lowest energy is in the tallest arrow, while the highest energy is near the arrowhead. However, there are a few exceptions to this rule from time to time.
- If we had to go higher than 4s, we can see that 4s has a lower energy state than 3d, according to the Aufbau principle.
Pauli-Exclusion principle
The Pauli exclusion principle is the second rule of ground-state electron configuration. Atoms are spin pairs that mean each orbital can hold electrons with opposite spins. Now we need to add electrons into 1s. Next, with the help of Aufbau’s principle, we can add electrons to each of the energy lines.
Hund’s rule
Hund’s rule is the third rule of ground-state electron configuration. Now, we can give one electron to each orbital when we have equivalent energy orbitals. However, we find the noble gas with a lower number on the periodic table than the atom we have.
Ground state electron configuration Ionization
If we use the aufbau principle correctly, it causes a reversal in the transition metals’ basic chemistry. The electron configurations of potassium and calcium are [Ar] 4s1 and [Ar] 4s2, respectively. It indicates that electrons filled the 4s-orbital before the 3d-orbital in the periodic table.
The 4s-orbital has n + l = 4 (n = 4, l = 0). On the other hand, the 3d-orbital has n + l = 5 (n = 3, l = 2). This follows Madelung’s rule, as the 4s-orbital has n + l = 4 (n = 4, l = 0). Most neutral atoms in the first series of transition metals (scandium through zinc) have two 4s electron configurations after calcium, with two exceptions.
The electron configurations of chromium and copper are [Ar] 3d5 4s1 and [Ar] 3d10 4s1, respectively. It indicates that one electron has migrated from the 4s-orbital to the 3d-orbital. This results in a half-full or filled subshell.
“Half-full or entirely filled subshells are unusually stable configurations of electrons,” is the standard explanation in this circumstance. The facts, however, contradict this, as tungsten (W) has a Madelung-following d4 s2 configuration. It does not have a d5 s1 configuration. Similarly, niobium (Nb) has an atypical d4 s1 configuration that does not give it a half-full or entirely filled subshell.
When electrons are taken from transition metal atoms to produce ions, the apparent paradox develops. The first electrons to be ionised come from the 4s-orbital, not the 3d-orbital. It is because one might assume if it were higher in energy. All atoms in the first series of transition metals have this electron exchange between 4s and 3d.
Ionization of the transition metals
The configurations of the neutral atoms (K, Ca, Sc, Ti, V, Cr, …) usually follow the order 1s, 2s, 2p, 3s, 3p, 4s, 3d,… However the successive stages of ionization of a given atom (such as Fe4+, Fe3+, Fe2+, Fe+, Fe) usually follow the order 1s, 2s, 2p, 3s, 3p, 3d, 4s, …
This surprising event is only seeming against common sense if it is took to be true. The power for a given time order of, being, like the smallest unit going round another is fixed and natural by the nuclear put payment through or by the existence of electrons in other going round another. If that were the example, the 3d-orbital would have the same power for a given time as the 3p-orbital. It is similar as in hydrogen. Yet it clearly does not in this case. There is no special reason why the Fe2+ ion should have the same electron form as the cr.
It is the smallest unit, given that iron has 2 more protons in its small group than cr, and that the chemistry of the 2 species is very different. Melrose and eric scerri have got at the details of the changes of going round another power for a given time with going round another work in terms of the two-electron disgust integrals of the Hartree-Fock careful way of, being, like the smallest unit structure answers by mathematics.
More recently scerri has argued on this. He said that opposite to what is stated in the sizeable greater number or part of starting points including the sign of position of his earlier unit on the thing talked of, 3d going round another rather than 4s are in fact most good taken.
Other Examples
In chemical environments, configurations can change even more: Th3+ as a bare ion has a configuration of [Rn] 5f1, yet in most ThIII compounds the thorium atom has a 6d1 configuration instead. Mostly, what is present is rather a superposition of various configurations. For instance, copper metal is poorly described by either an [Ar] 3d10 4s1 or an [Ar] 3d9 4s2 configuration, but is rather well described as a 90% contribution of the first and a 10% contribution of the second. Indeed, visible light is already enough to excite electrons in most transition metals, and they often continuously “flow” through different configurations when that happens (copper and its group are an exception).
Similar ion-like 3dx 4s0 configurations occur in transition metal complexes as described by the simple crystal field theory, even if the metal has oxidation state 0. For example, chromium hexacarbonyl can be described as a chromium atom (not ion) surrounded by six carbon monoxide ligands. The electron configuration of the central chromium atom is described as 3d6 with the six electrons filling the three lower-energy d orbitals between the ligands.
The other two d orbitals are at higher energy due to the crystal field of the ligands. This picture is consistent with the experimental fact that the complex is diamagnetic, meaning that it has no unpaired electrons. However, in a more accurate description using molecular orbital theory, the d-like orbitals occupied by the six electrons are no longer identical with the d orbitals of the free atom.
The ground state electron configuration of elements
The ground state electron configuration of Carbon
Carbon’s ground state configuration is the 1s2 2s2 2p2. All orbitals about an atom are regions where two paired electrons spend 95% of their time. However, these orbitals take many different shapes. Now, the reason they have different shapes is that we have a balance between the proton’s nucleus pulling the electrons in and the electrons repelling each other.
The ground state electron configuration of Sulfur
The ground-state electron configuration is one of the most well-known electron configurations, despite its simplicity. Sulfur is the fourth element in the block. We can find it in the third row of the periodic table. Sulfur has an atomic number of 16, which implies each sulphur atom has 16 electrons. List the atomic subshells in ascending order of primary and azimuthal quantum numbers. However, it requires 10 electrons to completely occupy shells 1 and 2. This implies that the remaining sex electrons should stay in shell 3. There is only one sub-shell in Shell 1. It can place only two electrons in the sub-shells.
Shell 2 now has two subshells with 1s 2s orbitals, where the next two electrons must be placed. We can accommodate Six electrons in a 2p subshell with three 2p orbitals. We may now begin filling 3, with the first two electrons entering the 3s orbital. Out of the two alternative configurations of 3p electrons, the remaining four must be placed in 3p orbitals because their energy is lower than that of 3d orbitals. The one with two unpaired electrons, on the other hand, is the one we prefer.
The ground state electron configuration of Phosphorus
We know that the atomic number of phosphorus is 15. Its electronic configuration in ground state is [Ne]3s23p3.
The ground state electron configuration of Nitrogen
7 is the number of protons, but it’s also the number of electrons. Nitrogen has 7 electrons, and we start with 1s. However, s orbitals can hold up to two electrons. So, we will put two for the number of electrons in the 1s orbital. Now we are down the 2s that will also hold two. Next, p can hold up to six electrons, but we need three more to complete the ground-state electron configuration for nitrogen. Now put 3 in that 2p orbital; we already have 2 plus 2 plus 3 that equals 7 electrons which is the number of electrons for the nitrogen. So, the configuration is 1s2 2s2 2p3.
The ground state electron configuration of Oxygen
The atomic number of oxygen is 8. The number of protons is equal to the atomic number, but it also equals the amount of electrons. We can quickly write the electron configuration for oxygen once we have the quantity of electrons. Because orbitals can now store up to two electrons, we’ll put it after the 1s. The 2s are next; we’ll add two more and have spent four electrons. Now we have the 2p, which can contain up to 6 electrons. We’ll need four more to finish the oxygen notation. After the 2p, we’ll just add a four. When we add those two numbers together, we get eight, which is the amount of electrons in oxygen.
The ground state electron configuration of Potassium
19 is the atomic number of potassium. Now we go down the first row, and we have 1s. Then we have 2s in the second row that can hold two electrons. Next, we go to the third row, and the first term is 2p. However, p orbitals can hold up to 6 electrons. So, we will put all 6 electrons in that 2p orbital. However, we have used a total of 10 electrons so far. Now we have 19 for potassium; also, in the third row, we have the 3s. Now, we will put two there and go for the next row, wherein the fourth row, we have 3p.
Next, we will put 6 electrons in the 3p; at this point, we have used 18 electrons. Now we only need one more to get to that 19. So after the 3p in the fourth row, we have that 4s turn. Next, all we need to do is put one electron there; 2 plus 2 plus 6 plus 2 plus 6 plus 1 gives us a total of 19 electrons. However, that is the ground-state electron configuration of potassium.
The ground state electron configuration of cr
The answer is 3d44s2.
3d subshell filled with 5 electrons (half-filled) is more stable than that filled with 4 electrons. 1, 4s electrons jumps into 3d subshell for more sability.
The ground state electron configuration of Boron
In writing the electron configuration for Boron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for B goes in the 2s orbital. The remaining electron will go in the 2p orbital. Therefore the B electron configuration will be 1s2 2s2 2p1.
Ground state Electron Configuration Of Fe3+
In writing the electron configuration for Iron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Iron go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We’ll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s are now full we’ll move to the 3p where we’ll place the next six electrons. We now shift to the 4s orbital where we place the remaining two electrons. After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d6.
Therefore the Iron electron configuration will be 1s2 2s2 2p6 3s2 3p6 4s2 3d6. Note that when writing the electron configuration for an atom-like Fe, the 3d is usually written before the 4s. Both of the configurations have the correct numbers of electrons in each orbital, it is just a matter of how the electronic configuration notation is written.
Therefore we have 1s2 2s2 2p6 3s2 3p6 3d6 4s2 For the Fe2+ ion we remove two electrons from 4s2 leaving us with: 1s2 2s2 2p6 3s2 3p6 3d6
For the Fe3+ ion we remove a total of three electrons (two from the 4s2 and one form the 3d6) leaving us with 1s2 2s2 2p6 3s2 3p6 3d5 1s2 2s2 2p6 3s2 3p6 3d5
The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds.
Ground state Electron Configurations For Copper (Cu)
In the 4s subshell, it will eliminate one electron. The electron configuration of the neutral Cu atom, on the other hand, is [Ar]3d10 4s1. Copper, as well as chromium, are two exceptions. A full d10 subshell has less energy. That is why copper prefers to fill the 3d subshell with 10 electrons before leaving the 4s subshell with only one electron. Because of this exception, the Cu+ ion’s electron configuration would require removing one electron from the 4s1 subshell. It resultingin [Ar]3d10.
Ground state Electron Configurations For Silicon (Si)
When adding electrons, the lowest energy levels are always filled first. This is shown by the Aufbau principle shown here-1s2 2s2 2p6 3s2 3p2.
FAQs on Ground state Electron Configuration
What is a proper ground state electron configuration?
1s2 2s2 2p6 3s1 is a reasonable configuration.
What element has a ground state of 1s2 2s2 2p6 3s2 3p6 4s2 3d7?
Nitrogen has a ground state of 1s2 2s2 2p6 3s2 3p6.
Which elements have the same ground-state electron configuration?
Cl and Ar are the elements that have the same ground-state electron configuration.
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6?
Antimony atoms with charge +2 have this configuration.
What is the correct electron configuration for neon?
[He]2s²2p⁶is the correct electron configuration for neon.
What do you mean by ground-state electron?
The lowest feasible energy configuration for an atom or molecule is known as the ground-state electron configuration.
What Is The Electron Configuration In The Ground State?
Let’s go over everything again. The ground state electron configuration is the arrangement of electrons with lower energy levels around the nucleus of an atom. The electrons in different energy levels’ orbitals naturally gravitate towards the lowest energy state, or ground state.
Why Is Electron Configuration Important?
The crucial point is that we recognise that understanding electron configurations aids us in determining the valence electrons on an atom. This is significant because valence electrons play a role in each atom’s unique chemistry. This is crucial when using orbital diagrams to describe an electron configuration.
What Is The Ground State Electron Configuration Of Magnesium?
The shell structure of magnesium atoms is 2.8.2. Magnesium atoms have 12 electrons. [Ne] is the ground state electron configuration of gaseous neutral magnesium. 3s2 is the term symbol, while 1s0 is the term symbol.
How Many Orbitals Are In Mg?
You already know that the atomic number of magnesium indicates the number of electrons in the element. A magnesium atom has 12 electrons, which means it has 12 protons. There are two electrons in shell one, eight in shell two, and two more in shell three, as seen in the diagram.
What Is The Ground State Electron Configuration For Aluminum?
Aluminum atoms have 13 electrons and the shell structure is 2.8.3. The ground state electron configuration of ground state gaseous neutral aluminium is [Ne]. 3S2. 3P1 and the term symbol is 2P1/2.
What is the Aufbau principle of ground state electron configuration?
The first rule of ground-state electron configuration is the Aufbau principle. Now we must start with the lowest energy states and work our way up to the higher energy ones. The 1s orbital is the first or lowest ground state. Next, write the energy levels 1 2 3 4 5 in our first column. The orbital names are s,p,d. We write them in the first row. The s orbital can carry one set of spin paired electrons, while the p orbital can hold three sets. The f orbitals, on the other hand, contain five sets of spin paired electrons. Finally, the d orbital can accommodate seven spin-paired electron pairs (14 electrons). S, on the other hand, is just a sphere that surrounds our nucleus. P orbitals come in three dumbbell-like shapes.
The p orbitals can carry 6 electrons because each dumbbell form can hold 2 electrons. The lowest energy is in the tallest arrow, while the highest energy is near the arrowhead. However, there are a few exceptions to this rule from time to time.
If we had to go higher than 4s, we can see that 4s has a lower energy state than 3d, according to the Aufbau principle.