So, anyone pursuing science would know that an atom has three parts. They are electrons, protons, and neutrons. So, when they work together, the atom is stable both in charge and in mass. Now, inside the atom, there are different parts in which these subatomic particles move about. Therefore, at the center of the atom is the nucleus. Hence, inside it, you can find both protons and neutrons. On the other hand, you will find electrons moving in the orbits that circle the nucleus. Now, it is also important to note that the neutron and proton hold each other by extremely strong forces. Therefore, when you try separating them, you create a nuclear fission reaction. Moreover, this is the mechanism behind forming an atom bomb. However, the question of the proton charge is important.

Cations and anions
Now, we already know that the atom is neutral in charge. So, this means that it neither has a positive nor a negative charge. Therefore how does this happen? So, the proton charge is positive. On the other hand, the electron charge is negative. Moreover, in a stable atom. The number of electrons and protons must be the same. Hence, the positive and negative charges are equal in an atom. However, an atom can gain and lose electrons because they are in the outer shells. So, when an atom loses an electron, there is an extra negative charge. Hence, the atom loses its stability and becomes negatively charged. This is called an anion. On the other hand, when an atom gains an electron, there is a lesser negative charge. Hence, the atom loses its stability and becomes positively charged. This is called a cation.
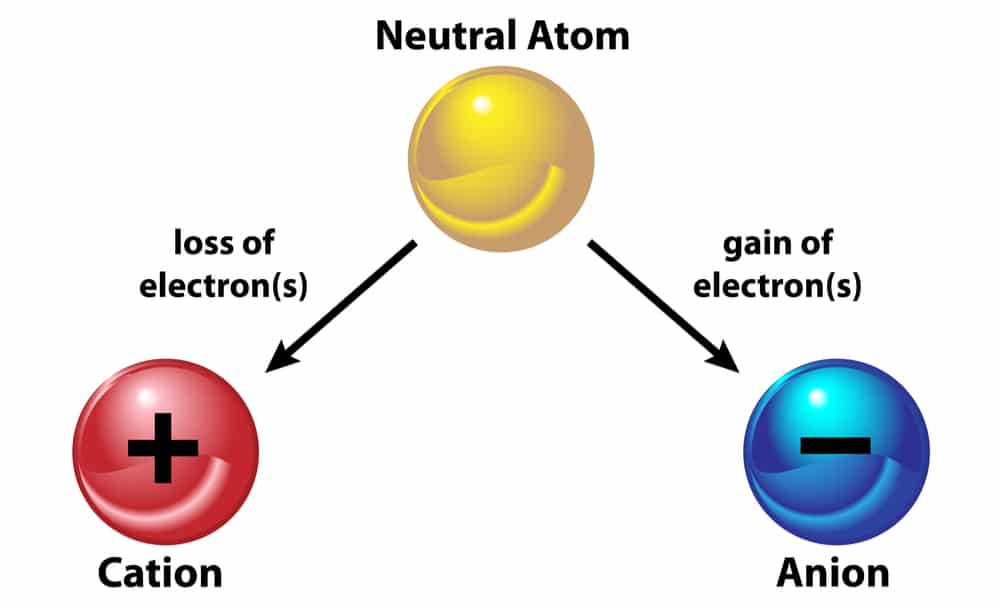
However, the proton charge always remains constant because protons are tightly attached to neutrons in the nucleus. So, they cannot leave it. Moreover, neutrons carry no charge.
Proton charge positive or negative
So, we already know that the positive charge in the atom comes from the proton. On the other hand, the negative charge comes from the electrons. However, the stability of the atom depends entirely on the presence of the electrons because unless there is a fission reaction, the proton charge always remains the same. It is also important that we realize that the electron or proton charge is not much dependent on the mass. So, protons and neutrons have the same mass. The mass of the nucleus, moreover, becomes the mass of the atom because the electrons have negligible mass.
Moreover, the masses and charges of these subatomic particles can give shape to several quantities. The number of protons that give the proton charge becomes the atomic number. So, make sure, the number of electrons in a stable atom can also give the atomic number but not when it is in an ion state. Moreover, the total number of protons and neutrons gives the mass number. However, there can be atoms of the same element with different mass numbers. These are what scientists call isotopes. Moreover, there can be elements of different elements with the same mass number. So, we call these isobars. Moreover, if atoms of different elements have the same number of neutrons, we call them isotones.

However, the atomic number is still the defining aspect of every element because of the stability of the proton charge. So, there might be isotopes but no two elements will ever have the same atomic number. Scientists have theoretically proven this. Now, let us see what is the value of the proton charge.
Proton charge value
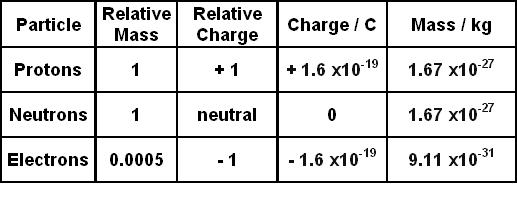
So, protons have a positive charge of +1. Therefore, the proton charge value is +1. Each proton in a nucleus adds a charge of +1 to the atom. It also has a unitary mass on the scale of amu. However, these might sound a little arbitrary. So, we can find out the magnitude of these quantities with the help of derivations. Therefore, we can find out the mass of a proton and the value of the proton charge in terms of various measurements. Let us see how.
Proton charge in Coulombs
So, coulombs is the SI unit of any electric charge. Therefore, it is the amount of electricity that passes in one second by a current of one ampere. Hence, when we find the magnitude of not just proton charge but that of any subatomic particles, we resort to coulombs.
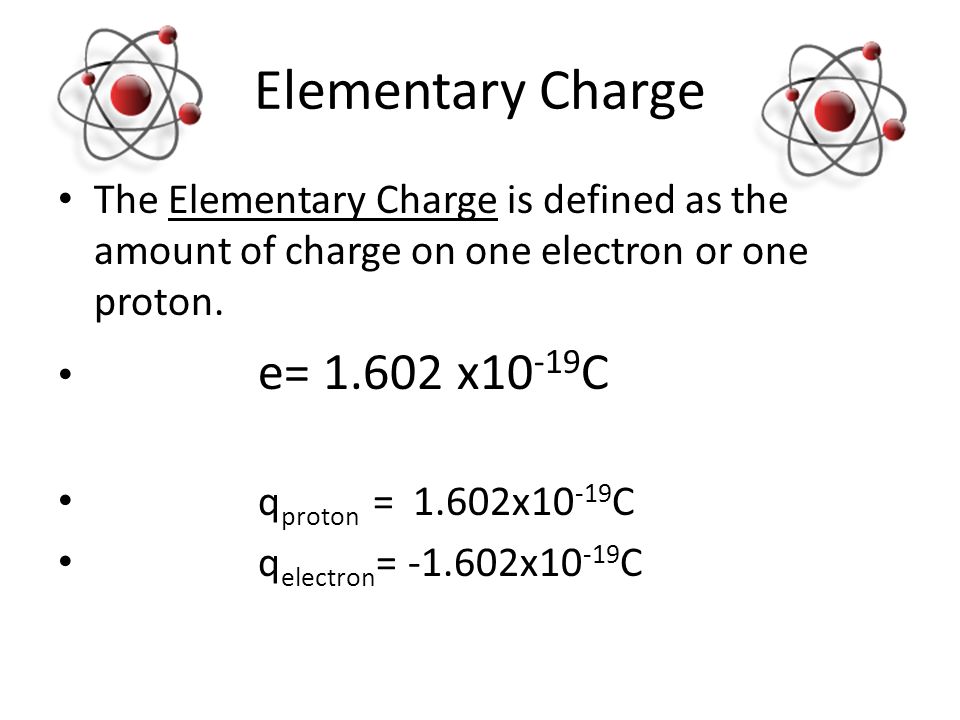
Therefore, now, the proton charge in coulombs is 1.602176634 × 10^ (−19) C. So, the symbol of coulombs is C. However, deriving this might be a little complex process as it is based a lot on delicate observations. Moreover, it is essential we realize that in use, the charge is (+1) x (1.602176634 × 10^ (−19) C).
But, once we know the value of proton charge in coulombs, we can make several other calculations. Let us say we have to calculate the total charge in coulombs that an ion carries. Therefore, we can simply find out the number of protons and electrons. So, after this, we will find out the difference between the two. Finally, if we multiply this by 1.602176634 × 10^(−19) C, we will get the total charge. However, it might be both positive and negative.
Moreover, 1 coulomb is the charge that 6.24 x 10^18 protons can carry. This is because if we do the unitary method, 1/ 1.602176634 × 10^(−19) gives 6.24 x 10^18.
Proton charge and electron charge
So, we already know that the proton charge and the electron charge are opposite in sign. However, they have the exact same value when it comes to the amount of charge. Moreover, this is crucial for the atom to be stable since we already know the number of electrons and protons inside an atom must be the same. Therefore, just like a proton, an electron also has a unitary charge but it has a negative charge of -1. However, the main difference it has with a proton is that it has so negligible mass that we do not even consider it. Therefore, the magnitude of the charge in coulombs for an electron is also 1.602176634 × 10^ (−19) C. However, in use we must multiply it by its charge, that is -1, otherwise, calculations might go all wrong.

Similarly, for proton charge also, we need to meticulously use the signs because without them the meaning changes, and the values we find out will be all messed up.
Proton charge in ESU
So, ESU is the electrostatic unit of charge. Therefore, just like coulombs, it is also a unit to measure charge. However, since we often deal with extremely small quantities at a subatomic level, using coulombs can be tricky sometimes. The calculations might be long and tedious. Therefore, for such calculations, we use the ESU unit. It is basically the cgs unit of charge. Moreover, we also call the ESU unit statcoulombs or franklin. Now, let us see the difference in values between ESU and coulombs.
So, the value of 1 ESU = 3.335640 × 10 ^ (-10) C.
Therefore, we know, 3.335640 × 10 ^ (-10) C gives 1 ESU.
So, 1 C gives 1/ 3.335640 × 10 ^ (-10) ESU.
Hence, 1.602176634 × 10^ (−19) C gives (1/ (3.335640 × 10 ^ (-10) )) x (1.602176634 × 10^ (−19) ) ESU.
Therefore, the calculation becomes a little complex. However, the final value you get out of here is 4.80320425 x 10^ (-10). So, 1.602176634 × 10^ (−19) C gives 4.80320425 x 10^ (-10) ESU. Moreover, 1.602176634 × 10^ (−19) C is the magnitude of 1 proton charge. So, 1 proton charge in ESU will be 4.80320425 x 10^ (-10) ESU. However, while using, remember to use the positive sign because it is the proton charge we are dealing with.
Proton charge and mass
So, we already know that the charge of a proton is +1 or unit positive and the mass of a proton is unit mass or 1 amu. Moreover, we also know the value of proton charge in ESU and in coulombs. Therefore, let us see the value of the mass of a proton.
Hence, the rest mass of 1 proton is 1.67262 × 10^ (−27) kg or 1.67262 × 10^ (−24) g. Moreover, this is the same as the mass of 1 neutron as they both have the same mass. However, unlike charge, the masses of protons and electrons are very different. So, let us also take a look at the mass of an electron.
Therefore, the mass of an electron is 9.1093837 × 10^ (-31) kg or 9.1093837 × 10^ (-28) g. Even a cursory glance would show how negligible the mass of an electron is in comparison to that of a proton. It is less than a fourth-order. So, roughly, the mass of a proton is 1,836 times the mass of an electron.

Proton charge to mass ratio
So, we already know the magnitude of the proton charge and the value of the mass of a proton. Therefore, we can simply place the values and find out the proton charge-to-mass ratio. We are using all SI units in this calculation.
Hence, the magnitude of the proton charge is 1.602176634 × 10^ (−19) C. Moreover, the mass of a proton is 1.67262 × 10^ (−27) kg.
So, the ratio of charge to mass is 1.602176634 × 10^ (−19)/ (1.67262 × 10^ (−27)) C/kg.
Therefore, this gives, (1.602176634 × 10^8)/ 1.67262 C/kg.
So, the final value comes out to be 0.958 x 10^8 C/kg. Therefore, we can also write this as 9.58 x 10^7 C/kg.
Hence, this is all that we can know about the proton charge and its mass before we start heading to calculations. However, the most important part is the sign and the units. Remember the values might change with respect to the units. So, if you are using the wrong value with the wrong unit, your entire calculation might go haywire.
Proton charge FAQs
What kind of charge does a proton have?
Ans. So, the proton charge is positive. It has a charge of +1. If there is an excess of proton charge due to a lack of electrons, the atom becomes a cation. To know more about the kind of charge proton has, scroll up and check the “proton charge value” section.
Do all protons have the same charge?
Ans. Yes, all protons have the exact same charge. So, if you calculate in coulombs this charge is
1.602176634 × 10^ (−19) C and in ESU, it is 4.80320425 x 10^ (-10) ESU. However, remember, it will always be positive.
Can you change the charge of a proton?
Ans. No, you cannot change the charge of any individual subatomic particle. So, the proton charge also remains constant. Moreover, you cannot even separate the proton from an element without a fission reaction. And in that case, if you do, the atomic number changes. Therefore, what you get is a completely new element.
Can a proton be split?
Ans. Yes, you can split a proton. However, it might need extreme high energy to separate the proton at first, and then again it is a very complex particle.
What is a proton made of?
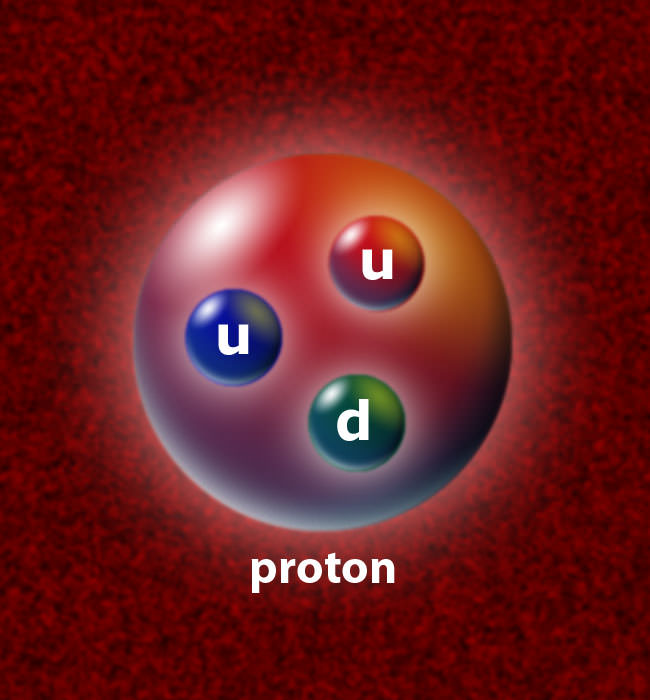
Ans. So, both protons and neutrons have two types of constituent particles: up quarks and down quarks. Therefore, each up quark has a charge of +2/3. On the other hand, each down quark has a charge of -1/3. So, the sum of the charges of these quarks that form a nuclear particle decides the electric charge that it will have. This is where the proton charge rises.
Where are protons found?
Ans. So, the protons stay together with the neutrons tightly bound in the nucleus. Moreover, the nucleus forms the center of the atom. This is where the mass of the atom is concentrated.
What particle has no charge?
Ans. So, neutrons have no charge. They are completely neutral and have the same mass as that of protons.
Do all protons have the same mass?
Ans. Yes, like proton charge, the proton mass also remains the same for all cases. To know more about this, scroll up and check out the “proton charge and mass” section.
What happens to the charge of an atom when it loses an electron?
Ans.So, when an atom loses an electron, there