We represent the chemical compound Sulfur Dioxide by the chemical formula SO2. The material is a colorless gas with a strong odor that resembles that of a burnt matchstick.
During volcanic eruptions, volcanoes eject a substantial amount of SO2. Some hot water springs also contain it. However, as a proponent of the greenhouse effect, sulfur dioxide contributes to global warming. The sulphur cycle, which also produces clouds of sulfuric acid on Venus, demonstrates this. We also found other bodies in the solar system with trace levels of the chemical.
People also make sulfur Dioxide commercially, roasting or burning sulphur and its constituents (sulphide ores, sulfites) in the presence of oxygen. However, through the contact process, they recover the released gas and use it largely in the manufacturing of sulfuric acid. We transform SO2 to Sulfur Trioxide. Then, it reacts with sulfuric acid to produce Oleum (disulfuric acid). Likewise, we can make Sulfuric Acid by mixing Oleum with water. To meet worldwide demand, people produce billions of kilograms of SO2 each year.
We can use SO2 as a preservative in dried fruits. We can also use it as a laboratory reagent, and in a variety of biological applications. It also has a widespread industrial use. It’s also being studied as a possible refrigerant and a climate engineering tool.
One must handle it with caution as an irritant. Long-term exposure might cause respiratory problems.
However, we shall go over the SO2 Molecular Geometry in great depth here. Sulfur Dioxide, commonly known as Sulphur Dioxide, is the result of a link between the atoms of sulphur and oxygen. One can write it as SO2 in a formula. SO2 molecular geometry, SO2 electron geometry, SO2 bond angle, and SO2 Lewis structure will all be discussed here.
SO2 Hybridization Structure
After determining the number of valence electrons accessible, we proceed to construct the Lewis structure for SO2.
The core atom will be sulphur, which is also the least electronegative component in the molecule. Then we place two oxygen atoms next to the sulphur atom in the middle. However, we use four valence electrons to create covalent connections between Sulfur and Oxygen atoms, out of a total of 18. We defined the configuration in the diagram below.

On the exterior, it utilizes the remaining valence electrons to fill the outermost shells of the Oxygen atoms. As shown below, this configuration leaves a lone pair on the central sulphur atom.
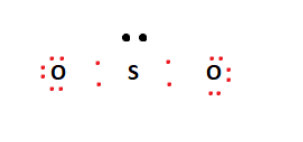
Finally, we met the octet criteria of the two oxygen atoms. However, this is not the case for Sulfur. Meanwhile, to overcome this, we build a double bond between one of the oxygen atoms and the central sulphur atom. Then, this double bond appears to fulfil the octet requirements of the sulphur atom. It resulted in the SO2 Lewis structure.

Stability of the SO2 Hybridization Structure
We can compute the formal charges of this Lewis structure to ensure its stability.
However, the most stable Lewis Structure state of an element/structure is determined by its formal charges. It is also calculated such that each atom’s elemental charge is as close to zero as possible.
FC = Valence Electrons – Nonbonding electrons – (Bonding electrons ÷ 2)
In this case,
| Element | V | N | B/2 | FC |
| S | 6 | 2 | 6/2 | +1 |
| Then, O | 6 | 6 | 2/2 | -1 |
| Then, O | 6 | 4 | 4/2 | 0 |
We depicted the formal charges below visually from the table above. However, any of the Oxygen atoms can participate in the creation of the double bond with the central Sulfur atom. Resonance structures develop as a result of this.
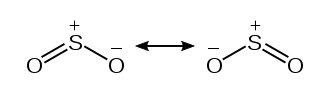
In theory, though, the formal charges would need to be as near to zero as possible. We can handle two double bonds by taking use of the Sulfur atom’s expanded octet. We defined the configuration in the diagram below.
Calculate the formal charges of the constituent atoms in this arrangement to ensure their stability.
In this case,
| Element | V | N | B/2 | FC |
| S | 6 | 2 | 8/2 | 0 |
| Then, O | 6 | 4 | 4/2 | 0 |
| Then, O | 6 | 4 | 4/2 | 0 |
However, the fact that the formal charges in the above table are all 0 means that the double-bonded SO2 arrangement is totally stable. This is also a theoretical structure that we derived from formal charges, and it is the Lewis structure of Sulfur Dioxide that we shall use.
Below is the final Lewis structure for SO2. It is obvious that it fulfilled all of the octet requirements. It bonded a lone pair to the centre Sulfur atom. Because of the Sulfur atom’s enhanced octet capacity, this is conceivable.
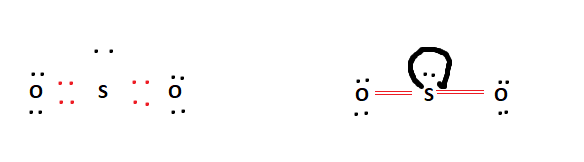
SO2 Hybridization Structure Mechanism
We call the electron positioning around the atoms of a chemical as Lewis structure. This structure also allows us to determine the type and amount of bonds that make up the compound.
Let’s have a look at how to draw a Lewis structure:
Step 1 – The first and most important step is to determine the total amount of valence electrons in the molecule. Take care of the + and – signs when doing so. A ‘+’ sign indicates that electrons are being lost, whereas a ‘-‘ sign indicates that electrons are being gained.
Step 2 – The next step is to identify the core atom. The centre atom has the most binding locations of all the atoms.
Step 3 – The next stage is to create a skeleton structure using only single bonds.
Step 4 – Following the establishment of single bonds, we work on establishing the octet of atoms with resting electrons. Begin with the electronegative atoms and work your way to the electropositive ones.
Step 5 – Giving double or triple bonds is essential if the octet rule is to be followed for all atoms.
Step 6 – Finally, it’s critical to determine whether all of the atoms have the lowest feasible formal charge.
The calculation of formal charges can be done using:-
Formal charge = [no. of valence electrons] – [electrons in lone pairs + 1/2 the number of bonding electrons]
The relationship between the amount of bonding electrons and how many are technically “maintained” by the atom is precisely stated in this formula.
When we apply this to BH4, for example, we get:
Boron has a valence electron count of three. There are no non-bonded electrons in the system. Around the boron, there are a total of 8 bonding electrons (full octet). 4 is one-half of this.
SO2 Hybridization Diagram
Now let’s see the Lewis structure of SO2.
Then, in SO2, the sulfur’s valence electron = 6
And the valence electrons of oxygen = 6
There are 2 oxygen atoms in the compound, thus = 6*2 = 12
So, total valence electrons = 18
We can see that none of the atoms can achieve their octet with single bonds after creating the skeletal structure. As a result, a double bond is required. As a result, the number of electrons used in double bonds is 8.
We have 10 electrons left after subtracting it from the total valence electrons. We must reposition the remaining electrons around the atoms as required.
Finally, the octet of atoms will be completed. Sulfur has one lone pair and oxygen has two.
Finally, make sure that all of the atoms have the same formal charge!
The next thing we need to know is about SO2 hybridization.
SO2 Hybridization Orbital Diagram
The molecular orbital diagram of SO2 is attached below:

The atomic orbitals of two separate atoms can fuse and give rise to a new orbital, as seen in a molecular orbital diagram.
This also aids us in determining the bond order, length, and strength of any molecule.
The AO of sulphur, which is on the left-hand side, combines with the AO of oxygen, which is on the right-hand side, in this MO.
The orbitals are filled with 18 electrons according to the proper rule.
Certain non-bonding orbitals can also be found there. In the case of SO2, the ant bonding orbitals are also vacant.
This concludes the discussion of SO2‘s molecular orbital diagram.
SO2 Hybridization Polarity
Because of the charge imbalance between the atoms in the SO2 molecule, it is categorized as a polar molecule.
Due to the fact that sulphur is more electronegative than oxygen, it attracts the charge to its side, resulting in a partial negative charge. As a result, polarization exists.
You can also read about the polarity of SO2 in this article.
Let us now turn our attention to the methods used to prepare it.
SO2 Hybridization Preparation
SO2 can be made in a variety of ways. I’m going to break down each strategy to make it easier to understand.
Method 1 – The main source of SO2 is during the contact process used to make sulfuric acid. This process is commonly utilized in industries, despite the fact that there are other ways to make SO2. (Because chemistry is so intertwined with history, here’s a fun fact.) However, the United States utilized 23.6 million tonnes of SO2 to make sulfuric acid in 1979.)
Method 2 – SO2 can be made by burning sulphur or sulfur-containing compounds.
S + O2 → SO2
Then, 2 H2S + 3O2 → 2H2O + 2SO2
Then, method 3 — The roasting of pyrite, sphalerite, and cinnabar can also produce SO2 (sulphide ores).
Method 4 – SO2 is also created as a byproduct during the manufacture of calcium silicate cement.
Then, 2CaSO4 + 2SiO2 + C → 2CaSiO3 + 2SO2 + CO2
Method 5 – SO2 is formed in the laboratory when hot concentrated sulfuric acid reacts with copper turnings.
Hence, Cu + 2H2SO4 → CuSO4 + SO2 + 2H2O
Then, method 6 – Natural disasters, such as volcanic eruptions, can release a lot of SO2.
Let us now turn our attention to the Lewis structure of SO2.
SO2 Hybridization
Sp2 is the hybridization of SO2.
Now, there are two methods to understand SO2 hybridization: one is through theory, and the other is through actual application of the formula. I would recommend understanding the theory first, then moving on to the formal.
A quick tip: when one s-orbital joins two p orbitals, the result is Sp2 hybridization with three equivalent orbitals.
In the case of SO2, the electrical arrangement of the ground state is 1s2 2s2 2p6 3s2 3p4. One electron from the 3px orbital transfers to the 3d orbital when in an excited state. As a result, we have 3p3.
The 3s2 and 3p3 now unite to create Sp2 hybridization, which has three analogous orbitals with two paired electrons and two unpaired electrons.

Sulfur requires two unpaired electrons from the Sp2 hybridized orbitals to create two sigma bonds with oxygen atoms. The lone pair of sulphur is formed by the remaining two paired orbitals.
Are you curious in the other two electrons in 3p that were not involved in the hybridization process?
Those two (one from the 3p orbital and another from the 3d orbital) produced the sulfur-oxygen bonds. For a better understanding, I’ve attached an image.
Now we’ll get to the formula.
SO2 Hybridization by Formula
The formula for finding hybridization of any compound is;
H = ½ [V+M-C+A]
Where,
- H depicts Hybridization
- V is the no. of valence electrons
- M is the number of monovalent atom present
- C depicts the cationic charge
- A depicts the anionic charge
If H is 2, the hybridization is Sp.
Sp2 hybridization occurs when H = 3.
Its Sp3 hybridization when H = 4.
Similarly, when H = 5 is used, Sp4 hybridization occurs.
Finally, when H reaches 6, Sp3d2 hybridization will occur.
Because oxygen is a divalent element, the number of valence electrons on the S atom is 6 and the number of monovalent atoms is 0.
Because this is a neutral molecule, the cationic and anionic charges will be zero.
Thus, H = ½ [6+0-0+0]
H = ½ * 6
H = 3 = Sp2 hybridization.
I hope the hybridization of SO2 is clear from both the explained concepts.
SO2 Hybridization and Shape
We must look at the Lewis structure of Sulfur Dioxide to figure out its molecular geometry. The centre Sulfur atom has two Oxygen atoms linked to it. A lone pair is also connected to the Sulfur atom. This suggested that the molecular shape was distorted.
To check and identify the molecular geometry of SO2, we can use the A-X-N idea and its table.
The letter ‘A’ stands for the centre atom. The value of ‘A’ is 1 in this case.
The number of atoms bound to the centre atom is denoted by the letter ‘X.’ Two oxygen atoms are linked to the Sulfur atom in this scenario.
As a result, X =2.
The number of lone pairs linked to the centre atom is denoted by the letter ‘N.’ Because there is a lone pair of electrons linked to the Sulfur atom, N = 1.
As a result, we’d have AX2N for the SO2 molecule. The molecular geometry can be determined using the A-X-N table below.
| Formula | Shape | Bond Angle (Theoretical) |
| AX2 | Linear | 180 |
| AX3 | Trigonal Planar | 120 |
| AX4 | Tetrahedral | 109.5 |
| AX5 | Trigonal Bi-pyramidal | 120, 90 |
| AX6 | Octahedral | 90 |
| AX2N | Bent | 120 |
| AX2N2 | Bent | 109.5 |
The AX2N formula corresponds to a bent molecular geometry.
Therefore, Sulfur Dioxide has a bent molecular geometry.
SO2 Hybridization Valence Electrons
We must first establish the number of valence electrons accessible in order to create the Lewis structure of Sulfur Dioxide. These valence electrons serve as the structure’s building pieces. They’re found in the atom’s outermost shell, where the nucleus’s force of attraction is the smallest. As a result, they may be able to break free and participate in bond development and exchanges.
Each of the compound’s constituent atoms contributes a certain number of valence electrons to the overall structure. SO2 is made up of two oxygen atoms and one sulphur atom.
Sulfur belongs to the periodic table’s group 6 (Chalcogens), having the electrical configuration [Ne] 3s23p4. As a result, the valence electrons contributed by the sulphur atom are 6 x 1 = 6.
Oxygen has a valency of -2 and possesses six valence electrons (group 6). The electrical configuration of oxygen is 1s2 2s2 2p4. As a result, the two Oxygen atoms contribute a total of 6 x 2 = 12 valence electrons.
As a result, the total amount of valence electrons accessible for the formation of [SO2] is 6[S] + 12[O] = 18 valence electrons.
SO2 Hybridization Bonds
The bond angle of SO2 is 120 degrees. One atom of Sulphur covalently connected to two atoms of Oxygen. It causes electron pairs to repel each other, resulting in a 120-degree angle. V-shaped or twisted molecule geometry is thought to exist in SO2. Sulphur dioxide’s electron geometry, on the other hand, is in the shape of a trigonal planar. However, it angled the three pairs of bonding electrons at 119 degrees. There are two double pairs bonded together, as well as one lone pair, giving it a bent shape.
SO2 Hybridization Sulfur Atom
The sulphur hybridization in SO2 is sp2. One lone pair of electrons and two bonding domains make up the sulphur atom. The molecular geometry is V- type in shape, bending, or angular, and the bond angle is less than 120°.
SO2 Hybridization Drawing
The type of hybridization that occurs in sulphur dioxide is sp2. To do so, we’ll start with the sulphur atom, which will be the core atom. This core atom had the connection with two oxygen atoms during the production of SO2, and their structure is O=S=O.
What is the SO2 Hybridization?
The type of hybridization that occurs in sulphur dioxide is sp2. To do so, we’ll start with the sulphur atom, which will be the core atom. This core atom has a connection with two oxygen atoms during the production of SO2, and their structure is O=S=O. There is one sigma and one pi link created between sulphur and the two oxygen atoms in terms of bonding. It can also accommodate one lone pair in the atom.
Let’s take a closer look at it. Sulphur’s ground state has six electrons in the outermost shell, and the first two shells are full. The 3p portal has four electrons, while the 3s orbital has two paired electrons. It now needs four unpaired electrons to establish four bonds (with oxygen). This causes the excited state in sulfur to form, in which one 3px electron jumps to the 3d orbital. There will be one unpaired electron in one 3d orbital and three in 3p orbitals when this happens. The electrons that make up the sigma bonds and the lone pair, on the other hand, are at different energy levels. When hybridization occurs, it obtains the stable state.
We hybridize two 3p orbitals and one 3s orbital during the process. There are a total of three sp2 hybrid orbitals forming. Two hybrid orbitals will have unpaired electrons, while the lone pair will be in one hybrid orbital. The oxygen atoms subsequently form sigma bonds with the unpaired electrons. The 3d and 3p orbitals, on the other hand, stay unchanged and play a role in the creation of pi bonds.
Intriguingly, the oxygen atom hybridization in this molecule is also sp2.
Similarities between Sulfur and Oxygen atoms
- Both O and S have the identical ns2 and np4 outer electrical arrangement.
- Normally, O and S are divalent.
- The elements O and S are non-metals.
- Both have an allopatric shape.
- Also, both react with the oxidation state of -2 when reacting with metals.
- Both create covalent compounds when reacting with nonmetals, such as H2O, H2S, CO2, and CS2.
Dissimilarities between oxygen and sulfur
- Sulphur has three allotropic forms, whereas oxygen has two.
- At room temperature, oxygen is a gas, whereas at room temperature, sulphur is a solid.
- Sulphur is not soluble in water and is only sparingly soluble in oxygen.
- Then, sulphur is combustible, and oxygen aids in combustion.
- Sulphur is also diamagnetic in nature, whereas oxygen is paramagnetic.
- While steam does not react with water, it does produce a small amount of hydrogen sulphide and sulphur dioxide as it passes through boiling sulphur.
- Sulfuric acid or nitric acid easily oxidizes Sulphur, whereas oxygen does not react with acids.
SO2 Hybridization Effects on Human
- Sulfur dioxide is a poisonous gas that is hazardous to human health.
- It can irritate the eyes, nose, throat, and lungs’ skin and mucous membranes.
- Then, its excessive amounts might irritate and induce inflammation in the respiratory system.
- Sulfur dioxide emissions in the air can lead to the creation of other sulphur oxides (SOx).
- SOx can create tiny particles when it reacts with other substances in the environment.
- However, these microscopic particles can enter deep into the lungs, and in large enough quantities, they can cause health concerns.
FAQs on SO2 Hybridization
What are the hybridization, shape and geometry of SO2?
SO2 has a bent form and is sp2 hybridized with trigonal planar geometry.
What is the bond angle of the sulfur dioxide molecule?
The sulphur dioxide molecule has a bond angle of 119°.
Which orbitals are involved in the hybridization of sulfur dioxide?
In the hybridization of sulphur dioxide, there is an involvement of 3p and 3s orbitals.
What kind of bonds does the sulphur dioxide molecule have?
We can find sigma and pi bonds in sulphur dioxide molecules.
Is SO2 bent or trigonal planar?
The electron-domain geometry of sulphur dioxide, SO2, is trigonal planar. This is due to the fact that it possesses three electron domains. Sulfur has six valence electrons, which form two single bonds with two oxygen atoms, and one non-bonding lone pair.
Why is SO2 bent and not linear?
There is a lone pair on the sulphur in sulphur dioxide, in addition to the two double bonds. To reduce repulsions, it separates the double bonds and the lone pair as much as possible, bending the molecule.
What is the vsepr of SO2?
Because sulfur’s vsepr geometry is trigonal planar, it bends (c2v). The third group is the lone pair.
What is the Lewis structure of SO2?
In molecules, there are two double bonds between sulphur and oxygen atoms. In the Lewis structure of SO2, each oxygen atom contains two lone pairs, while each sulphur atom has one.
Is sulphur atom sp2 hybridized?
The sulphur hybridization in SO2 is sp2. One lone pair of electrons and two bonding domains make up the sulphur atom.
What is the geometry of SO2?
Sulfur dioxide’s molecular geometry is a bent form. Sulfur dioxide has a “Sulfur to Oxygen” ratio of 1:2. However, the Sulfur atom and the Oxygen atom form two double bonds in the sulphur dioxide molecule. Meanwhile, in the molecule of SO2, there are 5 lone pairs of electrons. When we dissolve SO2 in water, it produces a weak acid solution.
What is hybridization of co2?
The sp hybridization type of carbon dioxide is the most common. However, two additional atoms create a bond with carbon, resulting in this sort of hybridization. It can use two double bonds or one single + one triple bond to make a bond.