Ionic compounds are ionic substances that are kept together by ionic forces. Of course, elements can actually gain or even lose electrons to form their closest noble gas state. The creation of ions for the closure of the octet (by either receiving or releasing electrons) aids in its security.
Metals end up losing electrons to fill their octet in a collision with non – metals, while non – metals get electrons to fill their octet. Ionic compounds are formed when metals and nonmetals react.
The size and scope of the anions and cations show the shape of an ionic complex. Granules, oxides, hydroxide ions, sulfides, as well as the vast bulk of inorganic salts are ionic compounds. Electrostatic interaction among oppositely charged ions binds ionic solids intact.

For example, Na ions pull chloride ions, and chloride ions lure sodium ions. As a result, a tri-lattice of parallel Na+ and Cl– ions is built. This is a sodium chloride flake. Because the amount of sodium ions is the same as the amount of Cl ions, the cube is chargeless. The ions are kept in the lattice by the electrostatic forces amongst them.
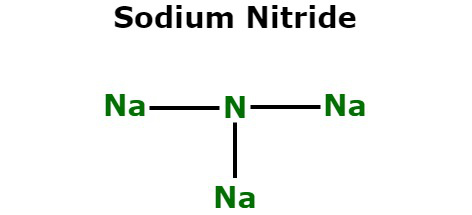
Similarly, Na3N is also a common Na3N ionic compound name.
Na3N ionic compound name
The inorganic chemical sodium nitride does have the molecular formula Na3N. The Na3N ionic compound name is quite common in the chemical sector. It is because it has a number of uses here. Na3N ionic compound, unlike lithium nitride and perhaps other nitrides, is indeed a very reactive alkali metal nitride. It can be made by mixing sodium and nitro atomic jets put on a minimal heated sapphire substrate. It swiftly decays into its constituents. The reaction looks something like this:
2 Na3N → 6 Na + N2.

The Na3N ionic compound can be made in two ways: heat decomposition of NaNH2 or by direct interaction of the atoms. Mr. Dieter Fischer and Mr. Jansen, as well as Grigori Vajenine, have used the latter way to generate huge Na3N. The first clause involves individually injecting precise Na and N2 quantities in gas form. And, putting them in a high vacuum on a cold base, which will then be warmed to room temp (298 K) to solidify.
Now, the second way is to use a metal surface to cause the interaction of elemental Na with nitrogen hyped to a plasma state. This formation can be aided more by adding liquid Na-K alloy with the compound, then scraping the extra liquid and cleaning with pure alloy.
Then, you can use a centrifuge to filter the grain from the fluid. However, Vajenine’s way is too air-sensitive and it can dissolve and burn fast unless left open to pure oxygen (O2).
AlCl3 ionic compound name
Very often, we know Aluminum chloride as aluminum trichloride. When Al and Cl react, it creates the product. AlCl3 is the chemical name for it. Aluminum chloride is mainly white in color and in look. However, because of the presence of impurities (iron(III) chloride), it gets yellowish.
Aluminum chloride is used in the making of Al metal. But, it also has many uses in the chemical sector, mainly as a Lewis acid. Aluminum chloride (AlCl3) is linked by covalent bonds. And, it also has a minimal melting and boiling range.
When subjected to temperature differences, this chemical molecule adopts a range of forms. It also hinges as to whether the item is in a strong solid, liquid, or vapor state.
AlCl3 has a cubed tight lattice form when it is solid. Its coordination shape would be octahedral in this event. In fact, AlCl3 exists as a dimer once it is fluid or molten. Its coordination shape would be tetrahedral in this context. Dimers split into trigonal planar shapes at extreme temps.
AlCl3 is a very strong Lewis acid.
You can use it in industries as a key chemical catalyst.
Also, AlCl3 is a harsh solid that is dry, non-explosive, and non-flammable. But, when it comes into contact with water or base, it burns quickly.
It is vital to monitor dry AlCl3 away from water and bases. Due to the sheer high heat of hydration, AlCl3 can erupt when it mixes with water. Also, it emits gasses into the air. During the redox process, safety gear such as eyewear, mittens, and face covers should be used.
Na3N ionic compound formula
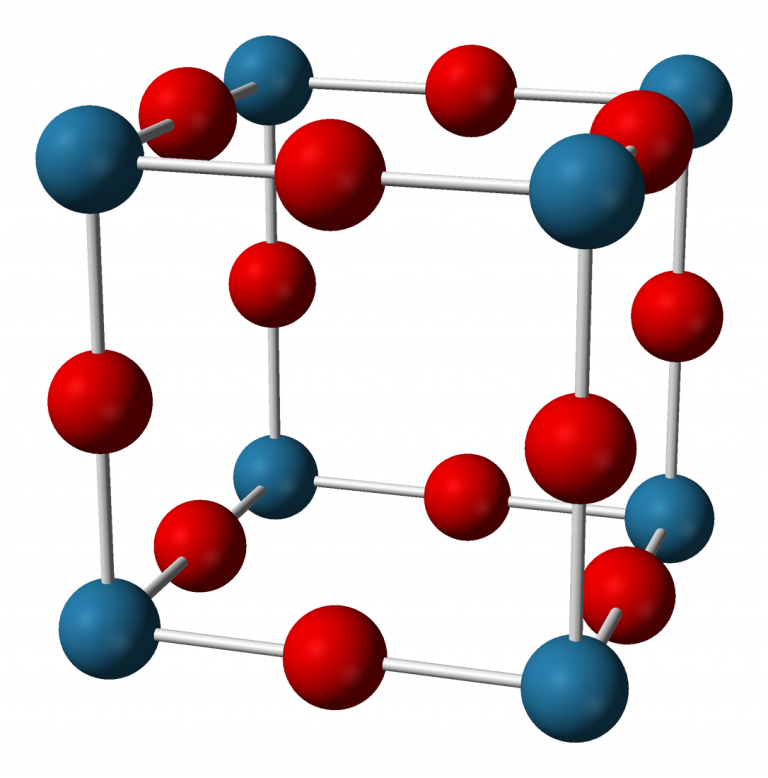
At room temp, the Na3N ionic compound is nearly 90% ionic. And, the energy gap is usual for a semiconductor. It has an anti-ReO form composed of a simple mesh of N-Na octahedra. The width of the N-Na link is 236.6 pm. Of course, you can use X-ray scattering to study the system shape. The hue of the chemical spans from dark red to navy blue, based on the synthesis method. It has no melting range.
Na3N ionic compound formula equation
Sodium Nitride, also known as Na3N, is an inorganic compound. You can make it by blending the atoms Sodium and Nitrogen set on a limited temp sapphire coating.
Sodium Nitride is a very unstable ionic material. You can make it by blending atoms and layering Na at a low temp. It has a density of 2.26g/mL. And, it is a white solid. You can widely use it as salt replacement and also as a food preservative. Sodium Nitride (Na3N) is an electrical inorganic compound with the formula Na3N. Collisions between metals and non – metals make all ionic materials .
To create this Na3N molecule, Na3N is an ionic composition of metals and nonmetals. Valence electrons in this ion compound moved from metal to A non-metal, which is why we get 3 Na atoms and then N. Further, In the periodic table, Na is in group I and has 1 valence electron. N is in Periodic Table Group number 15. And, it has five valence electrons in its shell. Since this is an ionic molecule, sodium will donate its oppositely charged ions to nitrogen, and give it a +1 charge. Now, nitrogen gets three valence electrons in its outer shell, resulting in a 3-charge. The ‘-‘ charge density pulls the positive electrical charge, leading to the formation of an ionic link.
Na3N ionic or covalent
Ionic bonding arises in Na3N ionic compound. In its valence shell, Na has a single electron. But, N has five. Three sodium ions lose the extra shell e- to make triple Na+ anions. N needs 3 electrons from every Na atom to make N3- by having to fill its outer electron shell. Both N and Na swap electrons to reach a balanced posture.
As a result, Na3N is indeed an ionic substance.
Na3N ionic compound name FAQs
1. What is the name of Na3N?
Ans: The IUPAC name for Na3N is sodium nitride. The values of component ions in ionic bonds need to balance. You can do this by using 3 na+ per nitride ion. As a reason, the formula for sodium nitride is Na3N.
Na3N, on the other hand, is very unstable. It decays quickly into basic elements.
2. What is the formula of the nitride ion?
Ans: Nitride ion’s formula is N-3.
A nitride is a N-based compound with the official oxidized state of 3. Nitrides are indeed a wide group of substances with many traits and uses. Since it is very basic, it hydrolyses almost instantly to form the nitride ion very fast. So, you can never find N3 in protic solution.
3. How do you write Na3N ionic compound?
Ans: The charges of individual ions in ionic bonds must equalize. This usually happens by using 3 sodium ions for every nitride ion. As a result, the formula for sodium nitride is Na3N.
4. Why is sodium nitride unstable?
Ans: Because Na3N is an inorganic chemical, it lacks carbon-hydrogen bonds. Sodium nitrides are very reactive alkali metals.
5. Why is sodium nitride solid at room temperature?
Ans: As a result, at ordinary room temperature, the power of these links is way more than the kinetic energy of the ion, and they cannot separate! The ions, on the other hand, secure tightly with each other in their orderly lattice structure. And, this is why they are a rigid solid under standard conditions.
6. Why do we use Na3N ionic compound?
Ans: You can use it as a feed additive and as a remedy to an overdose of cyanide. It acts as an antibacterial food preservative, an anti drug for hypertension, a food antioxidant, a toxin, and a poison antidote. It’s also a nitrite and an artificial salt of sodium.
7. Which bond is stronger, ionic or covalent?
Ans: Of course, this query has no universal answer.
- Many ionic bonds are more powerful than in others, and vice versa. In a space, ionic links are more tenuous than cross – links ( vacuum is a space which is devoid of all things, even air).
- Covalent links are generally more reliable than ionic forces in biological contexts (e.g., living cells). So, they are often watery (entail water). Because water can cause ionic compounds to split (like the breaking of salt in water).
8. Why can’t two sodium atoms form covalent bonds?
Ans: Sodium can bind via covalent linkages. But, they are so poor that they might arise only if the new Na2 molecule rarely gets in touch with anything at all. So, in fact, it is easier to argue it would never occur. However, We guess there might be a few Na2 molecules floating out there somewhere in space.
9. How to know if a sodium bonding is polar covalent or nonpolar covalent?
Ans: If the mutual electrons between molecules do not split equally, it forms a polar Covalent Bond. This arises when an atom has a stronger EN than the other with which it is swapping electrons. Of course, the hem with the greater EN will attract extra e-.
So, those with the greater EN will attract extra e-. As an end result, the mutual e- will be nearer to the one with the greater value of EN, resulting in uneven electronegativity. Because the common electrons shift toward the particle with the higher EN, a polar covalent relation induces the compound to have a mildly plus side (the side that encloses the particle with the lesser EN). Amd, also a gentle ‘-‘ side (the portion comprising the particle with the more EN value).
When atoms divide their negative ions equitably, a Nonpolar Covalent Bond forms. This often occurs when the two particles have near or equal electron attractions. The larger the force, the nearer their electron affinity scores are. This happens in gas particles, and we also call them diatomic elements. Nonpolar covalent bonds work the same way as polar covalent bonds. In fact, that is in how the element with the more EN value pulls the e- away from the poorer one.